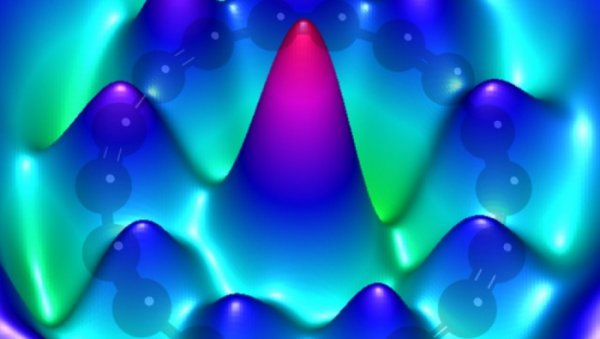
Carbon may be common in elemental macro schemes in our universe, but some of these forms may be even rarer-this is a challenge taken by the IBM Institute, which may change the electronics of the future. A team from the IBM Institute worked with Oxford University to create a so-called "ring carbon" ring for the first time. Carbon atoms are usually combined with the other 3 or 4 carbon atoms around them, at least in the most common form we encounter. For example, in graphite, each carbon atom is combined with another 3 carbon atoms. In diamonds, carbon atoms are combined with the other four. However, carbocyclic compounds reduce these pairings to two. In this way, they form a ring structure. Although they have been understood in theory for many years, due to their high level of reactivity-they tend to connect with another atom and destroy the ring structure-they have proved impossible to isolate. Three years ago, the researchers set out to try to change this situation. They used an inert surface to "cultivate" the carbocyclic ring at very low temperatures, and then imaged it using an atomic force microscope (AFM). However, even so, this is not a simple process. The IBM research team and the Oxford University team began to coat a relatively inert sodium chloride on top of the copper substrate. Due to the inert reaction, sodium chloride will not form a covalent bond with the top carbon atom. First, the team made a "linear segment", then built a ring of eighteen carbon atoms, stabilized by six carbon monoxide groups. After that, it was a matter of carefully removing carbon monoxide and hoping to keep the carbon atoms connected even without their “scaffoldingâ€. By applying a voltage pulse to the tip of the AFM, the carbon monoxide groups can be removed in pairs. As long as the surface of the substrate remains cool (approximately minus 450 degrees Fahrenheit)-the "ring carbon" ring is sufficiently stable to be studied. It allows researchers to solve a lingering question about formation: whether the ring is formed entirely from double bonds, or through alternating single and triple bonds. It turns out that the latter exists. However, it is not just theory that is being solved. By presentation. Atomic manipulation can fuse carbon rings and cyclic carbon oxides. Researchers have proposed that a large number of carbon-rich molecules and other forms can be created in the future. This may pave the way for molecular electronics. In molecular electronics, molecules are used to manufacture electronic components, but their scale is much smaller than traditional electronic components. In this way, new computers that use nanotechnology to break Moore's Law will one day not only be feasible, but also very practical. CHANGZHOU ANTALYA TOOL AND MACHINERY CO., LTD. , https://www.atly-tool.com