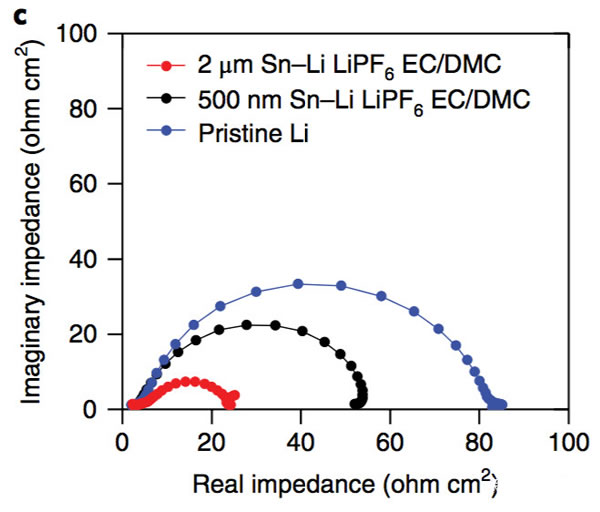
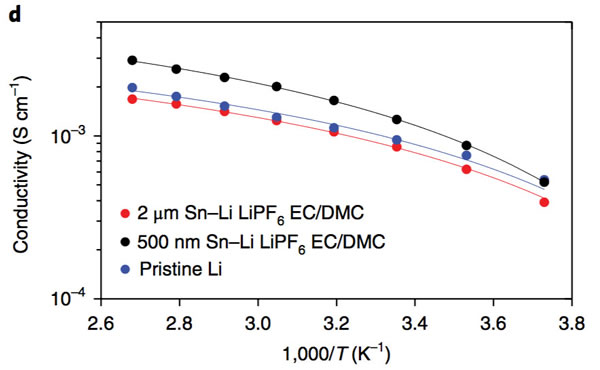
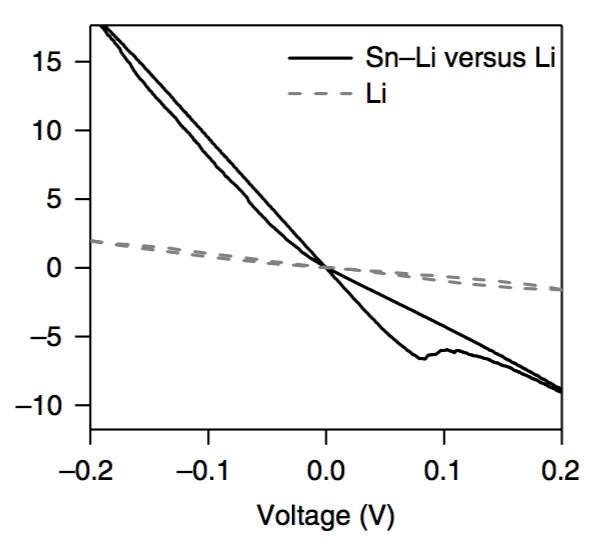
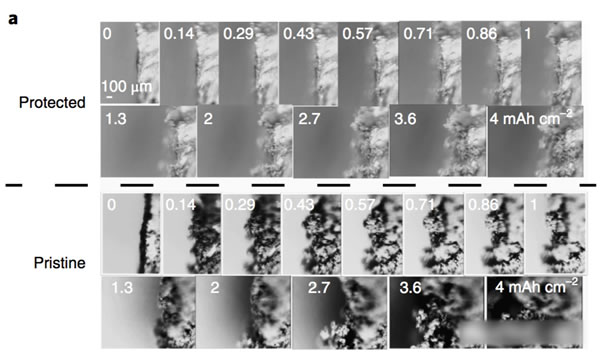
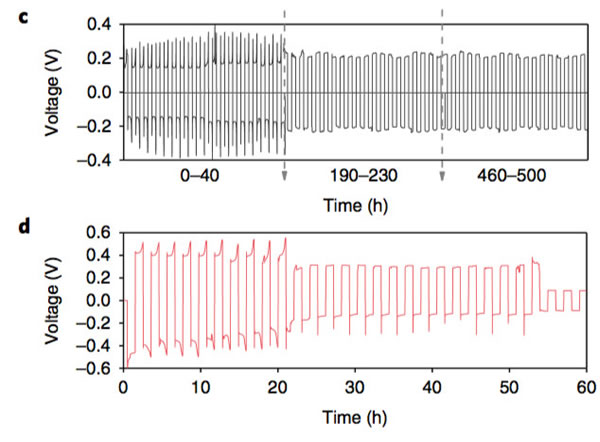
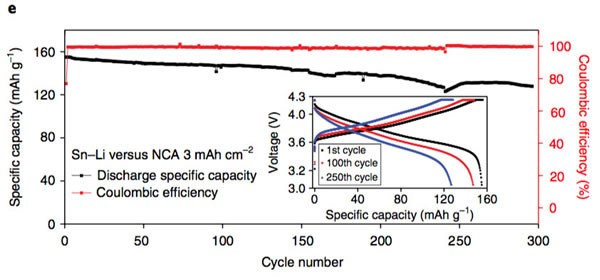
The key to improving the specific energy of lithium-ion batteries is positive and negative active materials. At present, the mainstream high-nickel ternary NCM and NCA materials, matching negative electrode silicon carbon materials can basically meet the requirements of 300Wh/kg, and even 350Wh/kg high specific energy batteries. Demand, but to further improve the lithium-ion battery specific energy to 400Wh/kg, even more than 500Wh/kg, the existing system can not do anything. From the current state of the art, the metal lithium negative electrode seems to be a very good option, its theoretical specific capacity is 3860mAh/g, the voltage platform is -3.04V (vs standard hydrogen electrode), and has excellent conductivity, Lithium-ion battery is very suitable for the negative electrode, people are not not tried, before the birth of lithium-ion batteries, the global chemical battery market once set off a wave of lithium metal secondary batteries, but in the end this attempt ended in failure The reason for this is that the Li dendrites generated during the cycling of metallic lithium negative electrodes may cause short circuits in lithium ion batteries, leading to serious safety problems. In order to solve the problem of lithium dendrites, people have also done a lot of work, and they have obtained a lot of research results from the electrolyte, artificial SEI membranes, as well as the production and growth mechanism of Li dendrites. This was also in our previous article. Made a lot of reports. Recently, Zhengyuan Tu et al. of Cornell University in the United States made a metal negative electrode with a composite structure by depositing a Sn element on the surface of an alkali negative electrode (Li, Na, etc.). This structure allows Li+ to diffuse rapidly on the surface of the electrode, thereby effectively The inhibition of the growth of Li dendrites greatly improves the cycle life of batteries using metal anodes. The synthesis process of the compound electrode used by Zhengyuan Tu is very simple. By adding the salt of the target metal element Sn in an ordinary carbonate electrolyte, the target metal element can be deposited at room temperature by ion exchange reaction on the surface of the metal Li anode. (As shown in the figure above), when talking about why Sn is used as a target element, Zhengyuan Tu said, “The reason why Sn was chosen as the target metal was mainly because Li diffused very fast in Sn and Li in Sn. The potential difference between the embedding and the detachment process is less than 500mV, which is conducive to the rapid diffusion of Li into the Li metal anode through the Sn layer. Zhengyuan Tu used the AC impedance tool to analyze the Li negative electrode after depositing Sn (the result is shown in the following figure c). From figure c below, we can see that the interface resistance of Li negative electrode after deposition of Sn has obviously decreased. The interface resistance of the negative electrode is approximately 80 W/cm2, and the interface impedance after deposition of 2 um Sn drops to about 25 W/cm2, which is more than a factor of three. In addition, we also noticed that after depositing a layer of Sn on the surface of Li, no extra semicircles were added to the EIS spectra. This means that the deposition of Sn on the surface of Li negative electrode did not increase the extra interface resistance. From the EIS data, the Sn deposition on the surface of the metal Li anode not only does not increase the impedance of Li+ at the electrode interface, but also because the existence of the Sn layer promotes the diffusion of Li+ at the interface, mainly because the metal Li is a very active material. Even if the metal is stored in argon, the surface will slowly grow an inert oxide layer, hindering Li+ charge exchange at the interface, and the deposition of Sn on the surface of the Li layer inhibits the surface of the lithium negative electrode Oxidation reduces the diffusion resistance of Li+ at the interface. The following figure d shows the relationship between the ionic conductivity and the temperature of the electrolyte in contact with different thickness Li layers of Sn-layers. It can be seen from the figure that the Li-anode of the 500-nm-thick Sn layer has the highest conductivity and the conductivity increases with temperature. Also showed a significant increase. The figure below shows the cyclic voltammogram of Sn-coated Li cathode and common Li cathode. Using Tafel formula to calculate the exchange current at the electrode interface, the exchange current of the Sn-Li composite electrode reaches 7.5mA/cm2, which is significantly higher. In ordinary metal Li electrodes, this is consistent with our previous results obtained from the EIS test. The presence of a Sn layer reduces the electrode interface resistance and accelerates the diffusion of Li+ at the electrode interface. The following figure shows Li deposition of Li-Sn negative electrode and common metal Li negative electrode at a current density of 4 mA/cm2. In the same figure, we can use the Li-Sn negative electrode (upper half of the figure below) to deposit the electrode during Li deposition. It is very smooth and there is no generation of Li dendrites. In contrast, the surface of ordinary lithium negative electrodes becomes very rough during deposition of Li, and Li dendrites begin to appear during continuous deposition. The role of Li-Sn composite electrode in inhibiting the growth of lithium dendrite can also be verified by the button cell test results. Zhengyuan Tu made two identical Li sheets into a button cell and repeatedly performed charge and discharge to verify Li for both negative electrodes. The characteristics of dendrite growth (the following figure is the Li-Sn electrode, the following figure d is the common Li electrode, the current density is 3mA/cm2, and the charge and discharge capacity is 3mAh/cm2). From the figure we can see that the common Li negative electrode is in the cycle. After 50 hours, lithium dendrites pierced the diaphragm, causing a short-circuit in the battery to cause a sudden drop in the battery voltage, while the Li-Sn battery was stably cycled for more than 500 hours without Li dendrite piercing the diaphragm. Zhengyuan Tu used the above-mentioned Li-Sn electrode and NCA electrode together to prepare a full battery, and the full battery also showed very excellent cycle performance. After 300 cycles, the capacity retention rate exceeded 80%, while the battery using ordinary metal Li anode was After several dozen cycles, it failed because of an internal short circuit. In addition, ZhengyuanTu's research also showed that deposition of Sn on the surface of Na negative electrode can also play an important role in inhibiting dendrite growth, significantly improving the cycle life of Na negative battery. Sn element has the ability of rapid Li diffusion, but due to the volume expansion during charge and discharge is too large, resulting in particle powdering and can not be applied, Zhengyuan Tu's alternative approach is to deposit Sn on the surface of the metal Li or Na negative electrode, not only make full use of Sn fast Li The ability to diffuse inhibits the growth of Li dendrites. At the same time, since Sn is directly in contact with the Li metal, it is always in a Li-rich state without violent volume expansion, thus stabilizing the Sn-electrolyte interface and reducing the SEI. The destruction and reconstitution of the membrane greatly improved the cycle stability of the Li, Na negative electrodes of alkali metals, opening up a new way for the application of metallic Li batteries. Steel Ball For Bearing,Carbon Steel Ball For Bearing,Ball Bearing,Stainless Steel Half Ball NINGBO YWC IMP. & EXP. CO.,LTD , https://www.nbywc-fastener.com