Region (or locus) selectivity is an important direction in the study of carbon-hydrogen bond activation. An organic compound often contains multiple similar carbon-hydrogen bonds, and how to accurately activate and functionalize the required carbon-hydrogen bonds is a key and urgent problem to be solved. Under the auspices of the National “Year for Thousand Talents Program†and the National Natural Science Foundation of China, the State Key Laboratory of Structural Chemistry of the Institute of Physical Structure Research, Chinese Academy of Sciences and the researcher of the coal-based ethylene glycol and related technologies key laboratory of the Chinese Academy of Sciences Li Gang’s group of researchers are in the international community. For the first time, olefination of multiple selective carbon-hydrogen bonds in the remote regions of phenylethylamines has been achieved (Figure 1). Many phenethylamines are very important drugs, such as the famous Amphetamine. The study succeeded in realizing the remote ortho and meta-position of phenylethylamines by selecting a suitable metal palladium catalyst system and designing a unique set of Directing Group for o-cyanobenzoic acid. (meta) Controllable multiplexed carbon-hydrogen bond olefination (Figure 1). And the selective control method is very simple, only one methylation reaction is needed, ie, the hydrocarbon olefinization from the remote ortho-position can be changed to the remote meta-hydrocarbon olefinization. Continuous use of selective hydrocarbon activation reactions in these two different regions can realize the rapid functionalization of carbon-hydrogen bonds in both remote and meta-positions, providing a completely new synthetic design concept for the construction of multi-substituted complex aromatic ring systems. Since only simple manipulation of the substrate structure is required, two different and predictable regioselective results can be obtained. This innovative synthesis reaction is of great importance for accelerating drug development and production synthesis. The related research results were recently published in "Chemical Science" (Chem. Sci. 2015, 6, 5595. doi: 10.1039/c5sc01737h). Based on the above studies, the team successfully controlled the meta-selective carbon-hydrogen bond activation of benzoic acid compounds with important practical value through the regulation of the positional cyano-functional groups (Figure 2). Benzoic acid derivatives are widely found in natural products and drug molecules. The use of metal-catalyzed hydrocarbon-activated methods for direct functional group transformations on aromatic rings of benzoic acid can greatly increase the efficiency of the synthesis and derivatization of such compounds. However, due to the electron-deficient nature of aromatic rings of benzoic acid, direct meta-carbon-bond functionalization reaction of this kind of reactants is often difficult and usually requires very harsh conditions. Previous successful examples of metal-catalyzed direct functionalization have also focused almost exclusively on the ortho-hydrocarbon bond activation of benzoic acid and its derivatives, and the direct functional group on the meta carbon-hydrogen bond of benzoic acid and its derivatives. Reaction, no more general and mild methods have been found before this study For the first time in the world, the research team designed a Directing Group that promotes the meta-carbon-hydrogen bond of the metal palladium-activated benzoic acid derivatives, and achieved direct olefination of meta-benzoic acid hydrogen bonds. Oxidation and subsequent series of various functionalized derivatization reactions. The reaction has a wide range of substrates, and oxygen can be used as an oxidizing agent in the olefinification, which avoids the disadvantage of using an expensive silver salt as an oxidant in a similar reaction. This study provides important foundations and ideas for the study of other meta-functionalization reactions of aromatic compounds such as benzoic acid. It has very important practical value for the efficient synthesis of bioactive natural products and efficient drug development. The above research results were published in Nature Communications (Nat. Commun. 2016, 7, 10443. DOI: 10.1038/ncomms10443). Wireless Temperature Transmitter Here you can find the related products in Wireless Temperature Transmitter, we are professional manufacturer of Wireless Temperature Transmitter,Remote Temperature Transmitter,Temp Transmitter,Temperature Indicator Transmitter. We focused on international export product development, production and sales. We have improved quality control processes of Wireless Temperature Transmitter to ensure each export qualified product. Wireless Temperature Transmitter,Remote Temperature Transmitter,Temp Transmitter,Temperature Indicator Transmitter Xi'an Gavin Electronic Technology Co., Ltd , https://www.gaimcmeaso.com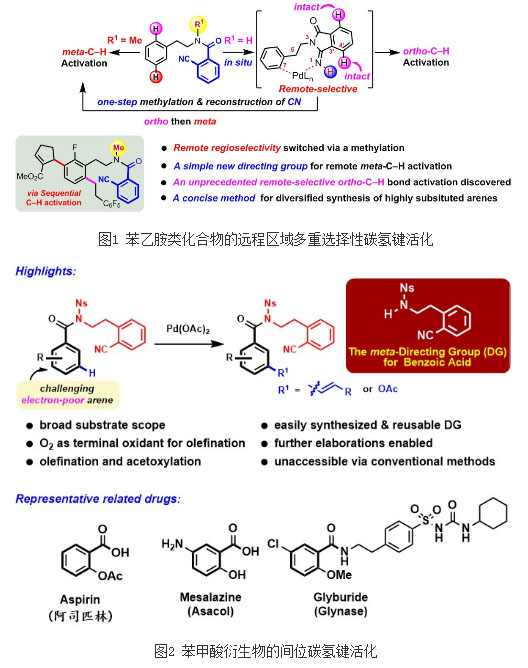
If you want to know more about the products in Wireless Temperature Transmitter, please click the product details to view parameters, models, pictures, prices and other information about Wireless Temperature Transmitter,Remote Temperature Transmitter,Temp Transmitter,Temperature Indicator Transmitter.
Whatever you are a group or individual, we will do our best to provide you with accurate and comprehensive message about Wireless Temperature Transmitter!