Brass Floor Drain,Brass Floor Waste,Brass Floor Drain Cover,Floor Drain Brass Zhejiang minmetals huitong import and export Co., Ltd , https://www.zjminmetals.com
Recent studies have shown that this form of corrosion exists not only in chrome steel, chrome-nickel steel, but also in nickel, copper, and aluminum-based alloys. The cause of intergranular corrosion is the chemical composition non-uniformity in the grain boundaries and in the crystal.
In stainless steel and nickel-based alloys, the mechanism of intergranular corrosion can be divided into three basic types: one is corrosion and the element that resists corrosion in the medium is depleted along the grain boundary zone; the second is corrosion and grain boundary The chemical stability of the precipitate is related; the third is that the corrosion is caused by the segregation of the surface active element which reduces the corrosion resistance of the matrix along the grain boundary.
The intergranular corrosion of austenitic stainless steel is mainly caused by the precipitation of continuous network-like chromium-rich (Cr,Fe)23C6 along the grain boundary in the sensitizing temperature range. Thereby, a chromium-depleted region is produced in the matrix around the grain boundary, and the width of the chromium-depleted region is about 10-5 cm. In the time when the precipitation of (Cr, Fe) 23C6 is not too long, the chromium-depleted zone cannot be recovered due to the slow diffusion rate of chromium. The formation of the chromium-depleted zone causes the chromium content near the grain boundary to be reduced below the n/8 amount limit, so that the chromium-depleted zone becomes a micro-anode and corrodes. If it is heated for a long time in the sensitization temperature range, the chromium-depleted region can be eliminated by the diffusion of chromium, and the intergranular corrosion tendency can be eliminated. 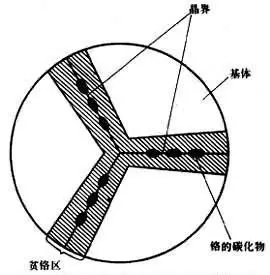
Since the corrosion resistance of steel is related to the carbide reaction, it is clear that the carbon content in austenitic steel and its thermodynamic activity determine the tendency of intergranular corrosion of steel. Regarding the carbon content, since the solubility of austenite in Cr18Ni9 steel at C ° C or lower C was 0.02%, almost no (Cr, Fe) 23C6 precipitated. In fact, intergranular corrosion does not occur when C ≤ 0.03%.
Therefore, the most effective way to solve the tendency of stainless steel intergranular corrosion is to produce ultra-low carbon stainless steel, so that C ≤ 0.03% in steel, such as 00Cr18Ni10 steel. For elements that affect the thermodynamic activity of C, all elements that increase the activity of C (such as nickel, cobalt, silicon) promote the formation of intergranular corrosion; any element that reduces the activity of C (manganese, molybdenum, tungsten, vanadium, niobium, titanium) ) all hinder the formation of intergranular corrosion. For this reason, a strong carbide forming element titanium or niobium is often added to the austenitic stainless steel to form a stable TiC or NbC, and C in the steel is fixed. Such as 1Cr18Ni9Ti, 1Cr18Ni11Nb and so on.
Steel has 10% to 50% by volume of δ ferrite, which can improve the intergranular corrosion tendency of austenitic stainless steel. Since δ ferrite precipitates between 500 °C and 800 °C, (Cr, Fe)23C6 precipitates on the δ phase side of the δ/γ phase boundary, which excludes precipitation at the austenite grain boundary (Cr, Fe). 23C6, and the diffusion coefficient of chromium in the δ phase is 103 times higher than that in the γ phase, and no chromium-depleted region is produced. It must be pointed out that not only the precipitation of chromium carbide causes chromium depletion at the grain boundary, but also the precipitation of chromium nitride and σ phase causes chromium deficiency in the grain boundary.
In summary, in order to prevent the tendency of intergranular corrosion of austenitic steel, the following measures can usually be taken in the composition design and heat treatment process of steel:
In the composition design of steel, on the one hand, the carbon content in the steel is reduced; on the other hand, a stable carbide forming element (Ti, Nb) is added to the steel to precipitate a special carbide to eliminate the intercrystalline chromium-poor region. To this end, the content of titanium or niobium in steel is: 0.8% ≥ Ti ≥ 5 (C% - 0.02%), 1.0% ≥ Nb ≥ 10 (C% - 0.02%). In addition, it is necessary to strictly limit the content of impurity elements such as nitrogen, phosphorus, silicon and boron in steel.
In the heat treatment process, the austenitic stainless steel is usually quenched (solution treatment) at 1050 ° C to 1100 ° C to ensure the content of carbon and chromium in the solid solution. Annealing of unstable steel, homogenizing austenite composition, eliminating chromium-depleted zone; for stable steel, converting chromium carbide into special carbide of titanium and niobium, ensuring corrosion-resistant solid solution containing chromium Level.